Global in vitro diagnostic market overview
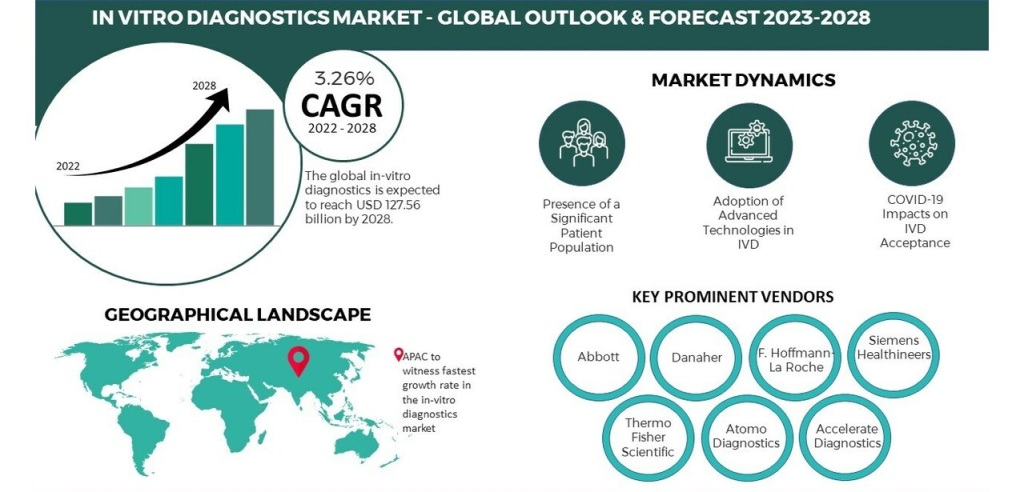
The global in vitro diagnostics market, valued at USD 132.95 billion in 2023, is on a trajectory of substantial growth, with a projected compound annual growth rate (CAGR) of -2.8% from 2023 to 2030. This growth is primarily attributed to the increased adoption of in vitro diagnostics market amid the pandemic and the development of automated IVD systems for more efficient and accurate diagnoses. Key players in the industry launching IVD products with molecular diagnostic capabilities have further fueled this upward trend.
Factors Driving Market Growth
The ongoing global health crisis has led to a surge in testing, propelling the demand for IVD Market. Automated systems designed for laboratories and hospitals are playing a crucial role in providing error-free and precise diagnostic results. The industry’s growth is also propelled by the introduction of molecular diagnostic products, which have proven to be effective and accurate in delivering results.
Innovations in Diagnostic Testing
In May 2021, the University of California developed an ultrasensitive molecular test based on chip technology capable of detecting influenza A and SARS-CoV-2 antigens. This innovative test is currently under study for conversion into a Point-of-Care (POC) test. A notable shift in industry dynamics has been observed, with an increasing focus on launching tests for home-based diagnostics. The FDA’s prioritization of home-based molecular diagnostic tests in 2021 further emphasizes this trend.
Challenges and Opportunities in the Market
While the benefits of early disease detection through in vitro diagnostics industry challenges are evident, the high prices of these tests pose a challenge, potentially encouraging patients to explore external substitutes. Despite this challenge, the market remains resilient, especially in the detection of newer infections such as SARS-CoV-2. The market landscape is also influenced by low penetration of IVD products from established players in certain regions, emphasizing the importance of local players offering cost-effective instruments.
Impact of Geriatric Population on IVD Industry

The rise in the geriatric population has contributed to an increased number of regular check-ups, considering that a majority of deaths due to infections and chronic conditions occur in individuals aged over 75 years. While healthcare costs have risen significantly, creating economic pressure on aging nations, this expenditure is anticipated to translate positively for the IVD industry, fostering market growth.
Product Insights
In 2022, the reagents products segment led the global market, accounting for over 65.00% of the revenue share. The segment is expected to maintain its dominance, growing at the second-fastest CAGR during the forecast period. This is attributed to extensive research and development initiatives by major market players focusing on the development of novel reagents. The launch of cancer testing kits, such as LiquidPlex Dx and FusionPlex Dx, in February 2022 by Invitae underscores the industry’s focus on niche, profitable areas in the IVD business.
Growth in Precision Medicine
The growth in precision medicine is expected to enhance the overall demand for novel reagents and consumables in the IVD market. Key players are aligning their instrument launches with the increasing global demand for genetic tests. For example, Thermo Fisher Scientific introduced the Ion Torrent Genexus Dx Integrated Sequencer in March 2022, with plans to develop assays for hemato-oncology and genomic profiling. This move is anticipated to further drive the adoption of the system in the market.
Technology Insights
In 2022, the molecular diagnostics application segment held the highest market share of 40.92%. Innovations in this segment, such as plasmofluidic chips, are contributing to ultrafast diagnostic capabilities. Plasmofluidic chips, as reported by ACS Nano Journal in May 2021, can perform a polymerase chain reaction (PCR) test in just 8 minutes, showcasing their potential to accelerate diagnosis in current and future pandemics. The immunoassay segment also experienced multiple launches in 2021, responding to the ongoing impact of the COVID-19 pandemic and the heightened consumer need for effective diagnostics.
End-use Insights
The laboratory end-use segment led the global market in 2022, accounting for more than 41.00% of the revenue share. This segment’s prominence is attributed to the increase in awareness about personalized medicine, a rise in demand for affordable services, and ongoing technological advancements. The higher accuracy of laboratory-based tests gives them a competitive edge over point-of-care (PoC) and home tests, contributing significantly to the segment’s market share. Hospitals, with a substantial market share of 36.44% in 2022, play a crucial role, especially in cases of hospitalizations that require support from faster diagnostics.
Conclusion
In conclusion, the global in vitro diagnostic market is on a robust growth trajectory, fueled by factors such as the increased adoption of IVD during the pandemic, technological advancements, and the development of innovative diagnostic products. The industry’s adaptability to home-based testing and the focus on precision medicine are contributing to its resilience. As the market continues to evolve, it stands as a vital pillar in global healthcare, offering efficient and accurate diagnostic solutions.
Read Also:- The Future of Healthcare: In Vitro Diagnostics Market Insights
FAQs
- What is the projected growth rate of the in vitro diagnostic market?
Ans. The market is projected to grow at a compound annual growth rate (CAGR) of -2.8% from 2023 to 2030.
- How do innovations like plasmofluidic chips impact diagnostic testing?
Ans. Plasmofluidic chips offer ultrafast diagnostic capabilities, performing a PCR test in just 8 minutes, potentially accelerating diagnosis in current and future pandemics.
- Why is the infectious disease application segment crucial in IVD?
Ans. The infectious disease application segment addresses life-threatening conditions and holds the highest market share, emphasizing its critical role in in vitro diagnostics.
- How does the rise in the geriatric population affect the IVD industry?
Ans. The increase in regular check-ups among the elderly contributes to the demand for IVD, especially as a majority of deaths due to infections and chronic conditions occur in this population.
- What initiatives are governments taking to promote homecare testing?
Ans. Governments globally are taking actions such as distributing COVID-19 tests to enhance testing rates and mandating insurance providers to cover the costs of homecare tests, fostering accessibility.